3,5-dinitrobenzamide 3,5-dinitrobenzamide
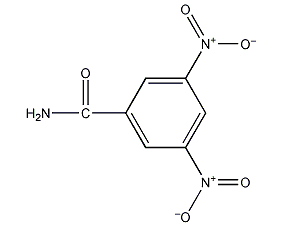

structural formula
| business number | 03e3 |
|---|---|
| molecular formula | c7h5n3o5 |
| molecular weight | 211.15 |
| label |
dinitrobenamide, nitromete, 3,5-dinitrobenzamide, nitramine, nitromid, nitromide, 3,5-dinitro-benzamid, benzamide, 3,5-dinitro-, component of tristat, component of unistat, component of unistat-3, tristat, fragrance |
numbering system
cas number:121-81-3
mdl number:mfcd00007985
einecs number:204-499-8
rtecs number:cv4752300
brn number:7096825
pubchem number:24893576
physical property data
1. melting point (℃):183℃
2. solubility:slightly soluble in cold water, slightly more soluble in hot water.
toxicological data
1, acute toxicity: rat oral ld50: 1250 mg/kg
rat transperitoneal cavity ld50: 320mg/kg
chicken orally ld50: 900mg/kg
chicken transperitoneal cavity ld50: 280mg/kg
turkey oral ld50: 1150mg/kg
ecological data
none
molecular structure data
5. molecular property data:
1. molar refractive index:48.27
2. molar volume(m3/mol):131.8
3. isotonic specific volume(90.2k):392.6
4. surface tension(dyne/cm):78.7
5. dielectric constant:
6. dipole moment(10(m3/mol):131.8
3. isotonic specific volume(90.2k):392.6
4. surface tension(dyne/cm):78.7
5. dielectric constant:
6. dipole moment(10-24cm3) :
7. polarizability:19.13
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 5
4. number of rotatable chemical bonds: 1
5. number of tautomers: 2
6. topological molecule polar surface area 135
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 272
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none
storage method
none
synthesis method
1. introduction to production methods
use3,5-prepared from the reaction of dinitrobenzoyl chloride and ammonium acetate.
purpose
2. purpose
used as anticoccidial drug. the advantage is that resistance develops slowly
= st1 />– 24cm3):
7. polarizability:19.13
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 5
4. number of rotatable chemical bonds: 1
5. number of tautomers: 2
6. topological molecule polar surface area 135
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 272
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none
storage method
none
synthesis method
1. introduction to production methods
use3,5-prepared from the reaction of dinitrobenzoyl chloride and ammonium acetate.
purpose
2. purpose
used as anticoccidial drug. the advantage is that resistance develops slowly
